Let 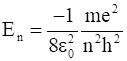 be the energy of the nth level of H-atom. If all the H-atoms are in the ground state and radiation of frequency (E2-E1)/h falls on it,
be the energy of the nth level of H-atom. If all the H-atoms are in the ground state and radiation of frequency (E2-E1)/h falls on it,
As in question it is mentioned that all the H-atoms are in the ground state so radiation of frequency (E2-E1)/h falling on it may be absorbed by some H-atoms and electron jumps to the next energy level i.e. first excited state (n=2). Now this frequency (E2-E1)/h is sufficient to take the atom from n=1 to n=2 state. So saying that all atoms will be excited to the n = 2 state is wrong hence option (c) & (a) are wrong and also option (b) is correct.
Now from above it is obvious that electron jumps from E2 to E1 by radiating the energy of same frequency i.e. (E2-E1)/h so electron from ground state will only jump to 1st excited state (n=2) and no atoms will jump to 2nd excited state (n=3). Hence option (d) is also correct.
Therefore option (b) & (d) are correct.