Deuterium was discovered in 1932 by Harold Urey by measuring the small change in wavelength for a particular transition in 1H and 2H. This is because, the wavelength of transition depend to a certain extent on the nuclear mass. If nuclear motion is taken into account then the electrons and nucleus revolve around their common center of mass. Such a system is equivalent to a single particle with a reduced mass μ, revolving around the nucleus at a distance equal to the electron-nucleus separation. Here μ = meM/ (me + M) where M is the nuclear mass and me is the electronic mass. Estimate the percentage difference in wavelength for the 1st line of the Lyman series in 1H and 2H. (Mass of 1H nucleus is 1.6725 × 10–27 kg, Mass of 2H nucleus is 3.3374 × 10–27 kg, Mass of electron = 9.109 × 10-31 kg.)
For first Lyman series, we have, ![]()
∴ ![]()
∴ for hydrogen,
![]() ------(1)
------(1)
And for deuterium,
![]() ------(2)
------(2)
Dividing 1 by 2 we get,
![]()
![]()
![]()
![]()
Now we know that,
![]() and
and
![]()
∴ ![]()
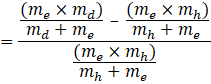
Now taking ![]() common from numerator, we get,
common from numerator, we get,
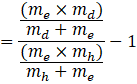
![]()
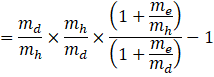
![]()
By binomial expansion we get,
∴ ![]()
![]()
![]()
Now putting the values respectively, we get,
![]()
∴ ![]()