If a proton had a radius R and the charge was uniformly distributed, calculate using Bohr theory, the ground state energy of a H-atom when (i) R = 0.1Å, and (ii) R = 10 Å.
In this problem we will calculate ground state radius and then then treat the nucleus as point or solid charged sphere.
Let the ground state radius in normal hydrogen atom be ![]()
From Bohr equation for angular momentum we have,
![]() --------(1)
--------(1)
And from force balance equation,
![]()
![]()
Now putting the equation 1 in the above equation we get,
![]()
![]() ---------(2)
---------(2)
On putting the values of charge and mass of electron and n=1, we get, ![]()
Case 1: since the r>R, nucleus is treated as point charge since electron will be outside the proton,
∴ ![]()
![]()
![]() ----------(3)
----------(3)
And ![]()
On putting the values we get, Total energy = K.E + P.E.
![]()
![]()
Case 2: since the radius r<R nucleus is treated as the solid sphere with electron orbiting inside it due to which there will be a new orbital radius of the electron at ground state r’,
Charge enclosed by the electron radius in the nucleus 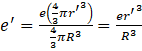
Force acting on the electron will be equal to the centripetal force,
![]()
![]()
Now, after replacing r with r’ in equation 1, putting it in above equation,
![]()
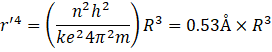
Putting the value of R, we get,
![]()
Now
![]()
![]()
![]()
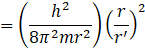
![]()
(using equation)
Using the formula for P.E. at point inside the solid charged sphere,
![]()
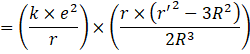
Where ![]() is the point inside the sphere and R is the radius of the sphere
is the point inside the sphere and R is the radius of the sphere
∴ on putting the values,
![]()
![]()
∴ total energy at ground state ![]()