The Bohr model for the H-atom relies on the Coulomb’s law of electrostatics. Coulomb’s law has not directly been verified for very short distances of the order of angstroms. Supposing Coulomb’s law between two opposite charge +q1, –q2 is modified to
![]()
![]()
Calculate in such a case, the ground state energy of a H-atom, if ε = 0.1, R0 = 1Å.
For ![]()
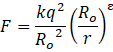
And we know by Bohr angular momentum formula,
![]() -------(1) where
-------(1) where ![]()
Now, the centripetal force keeps the electron revolving around the nucleus, ∴
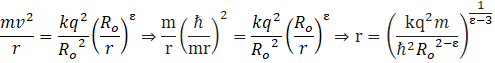
Now putting the values, we get,
![]()
This is the radius of the orbit of the electron in ground state
∴ ![]()
∴ ![]()
On putting the values, we get, ![]()
Now for P.E. ![]() from to r
from to r
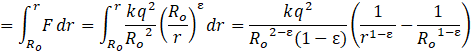
On putting the values, we get,
P.E.=![]()
For modified case,
total energy at ground state = ![]()
1