Samples of two radioactive nuclides A and B are taken. λA and λB are the disintegration constants of A and B respectively. In which of the following cases, the two samples can simultaneously have the same decay rate at any time?
The decay constant for A is ![]()
Therefore, the rate of decay is ![]()
Now the decay constant for A is ![]()
Therefore, the rate of decay is ![]()
Now let us check each option by putting the scenarios in the Decay law. From option (a) to (c) we can see that the initial decay rate of A and B are given. Let us first start by decay rate of A
The initial decay rate of ![]()
Putting the value in formula we get
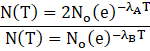
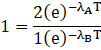
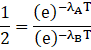
![]()
![]()
Therefore, putting the value of![]() , we get,
, we get, ![]() .
.
Hence, option B is true and A is false
In this option we saw that when the value of initial decay was changed to greater value the decay Constant also changed to higher value. Therefore, taking this consideration in mind if the value of initial decay of B is higher than A, then decay constant should also follow but that is not the case as option (c) says ![]() , hence, option (c) is false.
, hence, option (c) is false.
Last is the option (d) in which the time for the decay rate of A is ![]() and both have same initial decay rate. Therefore the decay constants are
and both have same initial decay rate. Therefore the decay constants are
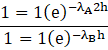
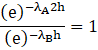
![]()
Therefore, the value of decay constant of A is greater than that of B. Hence, Option (d) is correct.