Sometimes a radioactive nucleus decays into a nucleus which itself is radioactive. An example is:
![]()
Assume that we start with 1000 38S nuclei at time t = 0. The number of 38Cl is of count zero at t = 0 and will again be zero at t = ∞. At what value of t, would the number of counts be a maximum?
Given
The reaction given in this scenario is
![]()
The number of ![]() nuclei = 1000, the initial time taken = 0, the number of
nuclei = 1000, the initial time taken = 0, the number of ![]() at time
at time![]() , half-life of
, half-life of ![]() and half-life of
and half-life of![]() . Hence, to find the value of time t when it is maximum, we use the relation of decay rate and number of live atom with respect to time.
. Hence, to find the value of time t when it is maximum, we use the relation of decay rate and number of live atom with respect to time.
Formula used
According to the Radioactive Decay Law or Rutherford Soddy Law the decay rate of a nucleus is given by the formula of
![]() ,
,
where
![]() is the rate of decay of the nucleus with respect to time,
is the rate of decay of the nucleus with respect to time, ![]() is the decay rate constant of the nucleus and N is the number of active nuclei.
is the decay rate constant of the nucleus and N is the number of active nuclei.
Explanation
The radioactive decay of Sulphur and Chlorine is given as
![]()
![]() The number of active Sulphur nuclei
The number of active Sulphur nuclei
![]() The decay rate constant of Sulphur
The decay rate constant of Sulphur
![]()
![]() The number of active Chlorine nuclei
The number of active Chlorine nuclei
![]() The decay rate constant of Chlorine
The decay rate constant of Chlorine
With the formula of radioactive decay in terms of initial and final number of atoms both before and after decay is
![]()
The rate of decay of active number of nuclei of Chlorine is
![]() _______________ (1)
_______________ (1)
Multiplying ![]() on both sides and integrating we get the sides as
on both sides and integrating we get the sides as
![]() ______________ (2)
______________ (2)
Now since at time![]() , the number of chlorine nuclei (
, the number of chlorine nuclei (![]() ) = 0. Therefore, the equation now stands as
) = 0. Therefore, the equation now stands as
![]()
The value of C = -![]()
Hence, putting the value of C in equation (2) we get:
![]() ______________ (3)
______________ (3)
To find the maximize value of the chlorine, we integrate the equation (3) on both side with respect to time and putting![]() , we get
, we get
![]()
Integrating, the above equation to find the value of t in terms of ![]() which leads to the time, when the number of nuclei
which leads to the time, when the number of nuclei ![]() is maximum
is maximum
![]() _____________________ (4)
_____________________ (4)
Now to find the value of time t, we put the value of ![]() in equation (4),
in equation (4),
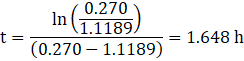
Therefore, the time at which the value of active nuclei of Chlorine will be maximum is 1.648 h.