On the basis of data given below predict which of the following gases shows least adsorption on a definite amount of charcoal?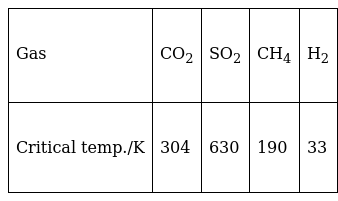
Critical temperature is the maximum temperature at which a gas can be liquefied by applying high pressure. Higher the critical temperature of a gas, more easily it can be liquefied and adsorbed.
Here, H2 gas has least value of critical temperature. So it shows least adsorption. So (iv) is correct.
Other gases, CO2, SO2 and CH4 have relatively higher values of critical temperature. So they show higher rate of adsorption. So options (i),(ii) and (iii) are wrong.
1