Which of the following phenomenon is applicable to the process shown in the Fig. 5.1?
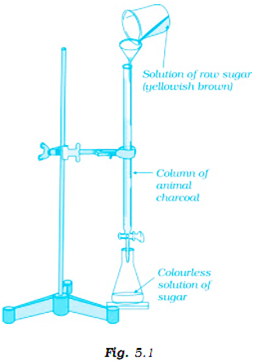
Here, absorption does not take place as it is a bulk phenomenon. In this figure, purification of raw sugar is done using the animal charcoal, which is a good adsorbent. So (i) is wrong.
Coagulation occurs when two oppositely charged ions get neutralized in presence of electrolyte. Here it is not applicable. So (iii) is also not correct.
Emulsification occurs between two immiscible liquids, which is not the case according to the figure. So (iv) is wrong.
In this figure, impurities present in raw sugar get adsorbed by animal charcoal. So this clearly indicates an adsorption process. So option (ii) is correct.
1