Method of formation of solution is given in Column I. Match it with the type of solution given in Column II.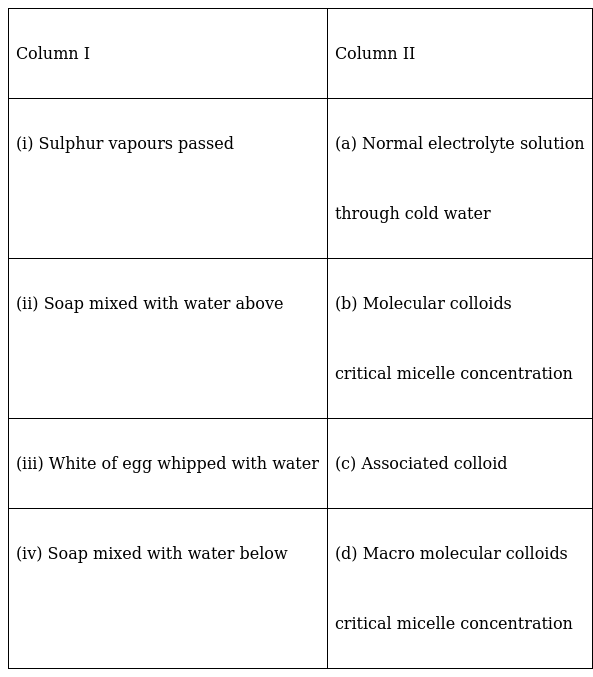
(i) – (b)
(ii) – (c)
(iii) – (d)
(iv) – (a)
EXPLANATION:
(i) Multimolecular colloids are those where the solution is formed as a result of the aggregation of a large number of atoms or small molecules (having diameters of less than 1nm) of the dispersed media. The dispersed particles are held together by Van der Val forces.
Example: Sulphur sol can be prepared by passing vapours of sulphur through cold water.
(ii) Associated colloids or micelles are those colloids which act like strong electrolytes at lower concentrations but at higher concentrations, they exhibit colloidal properties.
At a particular concentration, the molecules of dispersed phase align in such a way as to form micellar structures. This particular concentration is known as critical micellar concentration. Thus those colloids that form micelles are known as associated colloids.
So, when soaps are mixed with water, above critical micelle concentration, it leads the formation of associated colloids.
(iii) Macromolecular colloids are those which have very high molecular masses that form large molecules called macromolecules.
When these macromolecules are dispersed in a suitable dispersion medium, the resulting colloidal solutions are known as macromolecular colloids.
We know, proteins have high molecular mass and egg white contains proteins. So, when egg white is whipped with water, it becomes an example of macromolecular colloid.
(iv) Certain colloids behave as strong electrolytes at lower concentrations. So, when soaps are mixed with water below the critical micelle concentration, they form normal electrolytic solution.