Match the items of Column I and Column II.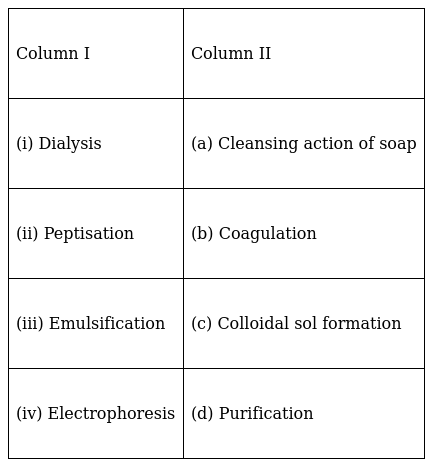
(i) → (d)
(ii) → (c)
(iii) → (a)
(iv) → (b)
EXPLANATION:
(i) Dialysis is the separation of suspended colloidal particles from dissolved ions or molecules of small dimensions (crystalloids) by means of their unequal rates of diffusion when they pass through a semipermeable membrane. So, this process is used in the purification of colloids.
(ii) Peptisation is defined as a process of converting a precipitate into colloidal sol by shaking it with dispersion medium in the presence of small amount of electrolyte.
It is the process used in the formation of stable dispersion of colloidal particles in dispersion medium. It is also called deflocculation.
(iii) An emulsion is a special type of mixture made by combining two liquids that normally don't mix (immiscible). It is a colloid of two or more immiscible liquids where one liquid contains a dispersion of the other liquids. This process of turning a liquid mixture into an emulsion is called emulsification. The process of cleaning and washing clothes is done by emulsification by removing oily or greasy dirt.
(iv) Electrophoresis is a separations technique that is based on the mobility of ions in an electric field. Positively charged ions migrate towards a negative electrode and negatively charged ions migrate toward a positive electrode.
So, it is basically the movement of charged colloidal particles under the influence of applied electric potential towards oppositely charged electrodes. They get discharged and precipitated. This process of setting of colloidal particles is called coagulation.