What is the role of adsorption in heterogeneous catalysis?
When catalysts exist in different phase than that of the reactants, it is said to be heterogeneous catalyst. These catalysts performing catalysis, is termed as heterogeneous catalysis.
Heterogeneous catalysis is also called surface catalysis. This is because generally, the catalyst is solid while the reactants are gases, due to which the reaction starts at the surface of the solid catalyst.
Eg.
• In the Contact process reaction (oxidation reaction of SO2 to SO3 using O2), solid V2O5 is used as a catalyst, whereas the reactants, SO2 and SO3 are gases.
![]()
• In Haber’s process, manufacture of ammonia gas is done by mixing nitrogen gas and hydrogen gas in presence of finely divided iron (which is a solid) as a catalyst at 400-450°C, and at 200 atm pressure.

• Hydrogenation of oils to form vegetable ghee using Ni as catalyst.
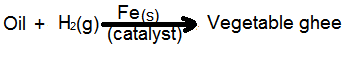
The mechanism and role of heterogeneous catalysis in increasing the rate of reaction is explained on the basis of process of adsorption :
(a) Old Adsorption Theory:
It stated that reactants in the gaseous state or in the solution state get adsorbed on the surface of solid catalysts. The rate of the reaction was increased with the concentration of the reactants on the surface of the catalysts.
It also told that adsorption is an exothermic process and heat of the adsorption is processed up in enhancing the rate of the reaction.
(b) Modern Adsorption Theory:
This theory is a combination of the old adsorption theory and the compound formation theory.
It tells that the catalytic activity is localised on the surface of the catalysts itself. The surfaces of the catalysts have free valencies which provide the main region of chemical forces of attraction.
The mechanism of catalysis involves these steps:
(I) Diffusion of reactant molecule to the surface of the catalyst.
(II) Adsorption of reactant molecules to the surface of the catalyst.
(III) Formation of an intermediate by the reaction of a chemical reaction on the surface of catalyst (due to the free valencies present on them).
(IV) Desorption of the reaction products occur from the surface of the catalyst. Now, this catalyst is available for more such reacting molecules.
(V) The reaction products diffuse away from the surface of the catalyst.