What do you understand by shape selective catalysis? Why are zeolites good shape selective catalysts?
The catalytic action that depends on the pore structure of the catalyst and the size of the reactant and product molecules is known as shape selective catalysis.
Zeolites exhibit shape selective catalysis due to the honeycomb-like structure, having a ‘truncated octahedra’ as their building blocks. Zeolites are microporous aluminosilicates having a general formula of Mx/n [(AlO2)x(SiO2)y].mH2O where n is the valency of the metal cation, Mn+. Some of the silicon atoms are replaced by aluminium atoms, thereby giving a Al-O-Si framework.
Zeolites to be used as a catalyst are heated in molecules so that the molecules of hydration are pushed out. This causes formation of cavities in the network structure and it becomes porous. The pores in zeolites range from 260-740 pm.
Thus, molecules having a pore size more than this cannot enter the zeolite and undergo the reaction and small sized molecules are absorbed in the pores and cavities of zeolites.
Zeolites are widely used as catalysts in petrochemical industries for isomerism and cracking of hydrocarbons.
One prominent example of zeolite used in petroleum industry is ZSM-5(Zeolite Sieve of Molecular porosity-5). It is used in the direct conversion of alcohols to gasoline (petrol).
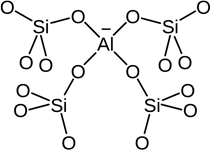
Structure of zeolite.