Ethers can be prepared by Williamson synthesis in which an alkyl halide is reacted with sodium alkoxide. Di-tert-butyl ether can’t be prepared by this method. Explain.
Ethers can be prepared by Williamson synthesis in which an alkyl halide is reacted with sodium alkoxide. Di-tert-butyl ether can’t be prepared by this method because in this case, elimination is more favoured over substitution. In other words, elimination competes over the substitution unlike in case of primary alkyl halides.So, alkene is formed as the major product and ether is not formed.
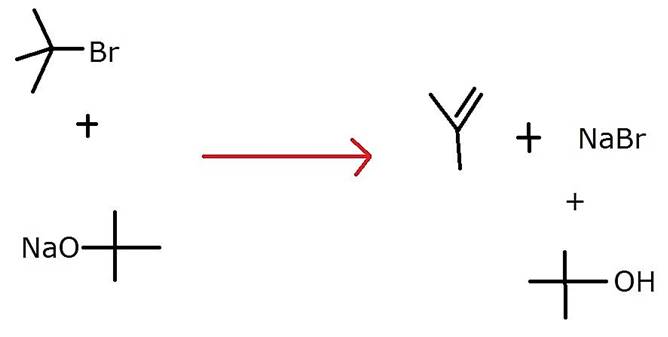
The major product formed in this reaction is 2-Methylprop-1-ene, an alkene and not ether.
1