Match the items of column I with items of column II.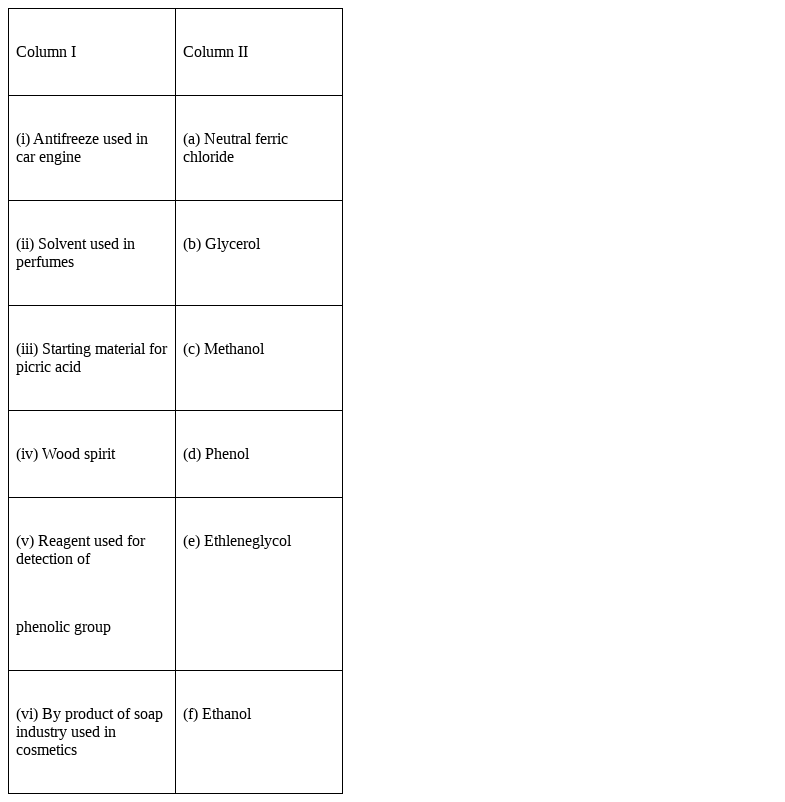
(i) Antifreeze used in car engine - (e) Ethleneglycol
Antifreeze are substances which when added to water lowers the freezing point. Thus they are mostly used in the radiators of a motor vehicle to prevent them from freezing in winters.
Ethylene glycol or ethane-1,2-diol is used as an antifreeze in car engine. It is used as an coolant in winter for car radiators due to its lower freezing point than water.
In summers also, ethylene glycol acts as a coolant to the car radiator and absorbs the heat.
![]() Ethylene glycol
Ethylene glycol
(ii) Solvent used in perfumes – (f) Ethanol
Ethanol (C2H5OH) is a good solvent for fatty and waxy substances. They are the main carriers of fragrance oils. When ethanol comes in contact with skin, it gets warmed by the temperature and gets evaporated quickly thereby releasing the fragrance evenly. Moreover, it is not so irritating to the skin. Thus they are used as solvent used in perfumes and so they are known as perfumers alcohols.
(iii) Starting material for picric acid – (d) Phenol
Phenols give 2,4,6-trinitrophenol(Picric acid) when it reacts with a mixture of conc. HNO3 and H2SO4. This is because of the production of negative charge on the three locations of the benzene ring- ortho and para (as shown in the diagram) due to the strong activating effect(+R effect) of the –OH group (due to the presence of lone pairs on Oxygen atom) on the benzene ring towards electrophilic reaction where the electrophile(-NO2 ) can easily attack.
Such is the effect of –OH group on benzene that instead of producing mono nitro derivative on the ring, the reaction readily proceeds to form tri nitro derivative that is 2,4,6-trinitrophenol.


(iv) Wood spirit – (c) Methanol
Methanol (CH3OH) is toxic in nature. It is called wood alcohol or wood spirit because earlier it was produced chiefly by the destructive distillation of wood. But today, it is produced industrially by the hydrogenation of carbon monoxide gas.
(v) Reagent used for detection of phenolic group – (a)Neutral ferric chloride
The test for the detection of phenolic group with neutral ferric chloride(FeCl3) is known as Ferric chloride test.
Phenol gives a violet-coloured water soluble complex with ferric chloride. The complex formed is a coordination compound in which iron is hexavalent.
![]()
In general all compounds having the enolic group ![]() (having ene and -ol groups) respond to this test.
(having ene and -ol groups) respond to this test.
(vi) By products of soap industry used in cosmetics – (b) Glycerol
Glycerol(propan-1,2,3-triol) is a thick, transparent liquid that is commonly found in soaps. It is a bye product formed during the saponification(process where soaps are produced) process. It is also used in cosmetics.
