Note : In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion : Phenol forms 2, 4, 6 – tribromophenol on treatment with Br2 in carbon disulphide at 273K.
Reason : Bromine polarises in carbon disulphide.
As we discussed earlier that in the presence of bromine water how phenols form a tribromo derivative of 2,4,6-tribromophenol.
What we told was in case of bromination of phenol , we do not need any Lewis acid. This is because of the strong activating effect(+R effect) of the –OH group (due to the presence of lone pairs on Oxygen atom) on the benzene ring towards electrophilic reaction. Thus Lewis acids are not required here to produce electrophiles.
Such is the effect of –OH group on benzene that instead of producing mono bromo derivative on the ring, the reaction readily proceeds to form tri bromo derivative that is 2,4,6-tribromophenol which is observed as a white precipitate when the reaction happens with bromine water .
Now, if we treat phenol with carbon disulphide(CS2) at 273 K, the reaction is somewhat suppressed and instead of a tribromo derivative, a monobromo derivative is produced because CS2 is a less polar solvent as compared to bromine water. p-bromophenol is produced as a major product and o-bromophenol is also produced but as a minor product.
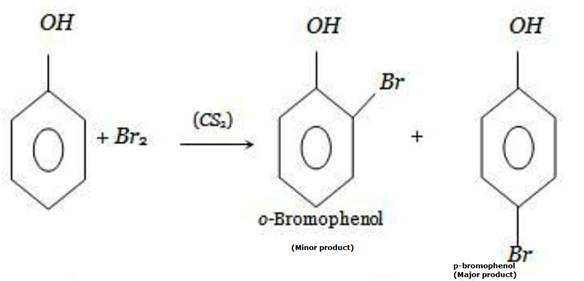
In phenol, polarization of bromine molecules take place even in the absence of Lewis acids.