Match the items of Column I and Column II.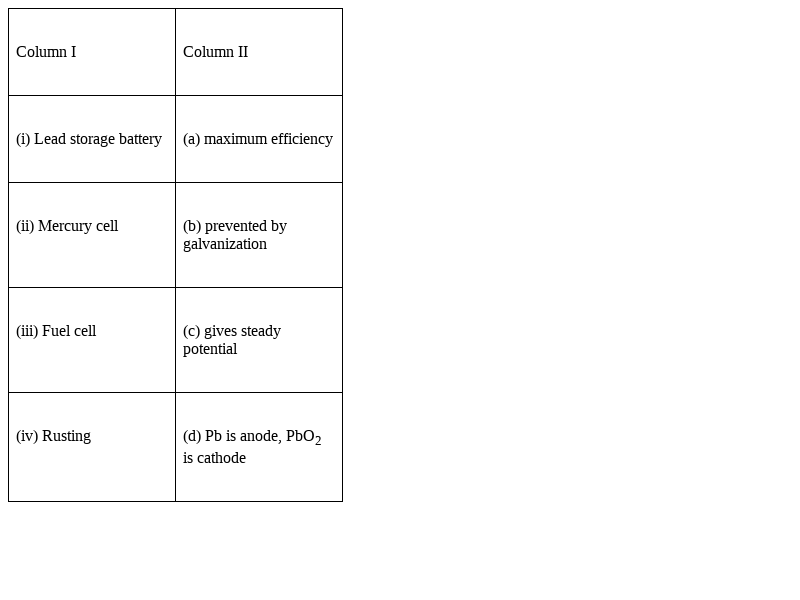
(i) Lead storage battery → (d) Pb is anode, PbO2 is cathode
(ii) Mercury cell → (c) gives steady potential
(iii) Fuel cell → (a) maximum efficiency
(iv) Rusting → (b) prevented by galvanization
(i) Lead storage battery is an example of secondary battery. It consists of a Pb anode and cathode made up of Lead dioxide.
(ii) The overall reaction of Mercury cell is represented as
Zn(Hg) + HgO(S) → ZnO(S) + Hg(l)
Mercury cell is a primary cell and the cell potential value is ~1.35V and remains constant throughout its life as they do not involve any ion whose concentration varies during its lifetime. Thus, gives a steady potential throughout its life.
(iii) Fuel cell uses Hydrogen and Oxygen to produce water molecules. The overall reaction is represented as
2H2(g) + O2(g)→ 2 H2O(l)
These cells run continuously until the reactants are depleted. They produce electricity with a higher efficiency value as compared to that by the thermal plants.
(iv) Rusting is basically the corrosion of iron in presence of air and water, where Iron is oxidized by loss of electrons to O2(g) and forms Iron oxide. But rusting can be prevented by galvanization, by applying a protective Zinc coating on the Iron.