Match the items of Column I and Column II.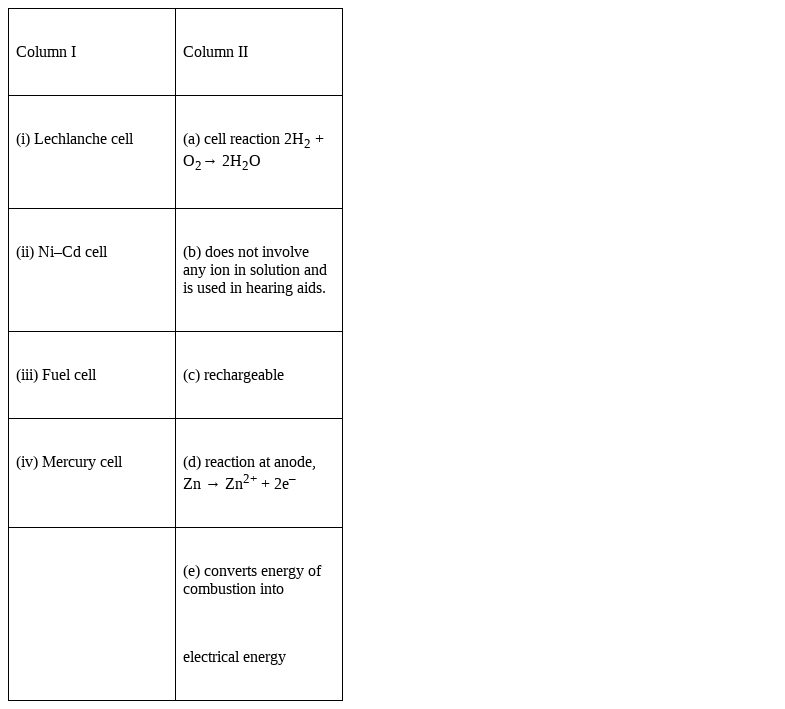
(i) Lechlanche cell → (d) reaction at anode, Zn → Zn2+ + 2e–
(ii) Ni–Cd cell → (c) rechargeable
(iii) Fuel cell → (a) cell reaction 2H2 + O2→ 2H2O ,
(e) converts energy of combustion into electrical energy
(iv) Mercury cell → (b) does not involve any ion in solution and is used in hearing aids.
(i) Lechlanche cell consists of a Zinc anode. The chemical reaction involved at anode is represented as:
Zn(S) → Zn2+ + 2e–
And the chemical reaction involved at cathode is represented as:
MnO2 + NH4+ + e-→ MnO(OH) + NH3
(ii) Ni-Cd cell is a type of secondary cell and is discharged after some time. But the good thing is that it can be charged again and therefore, they have a longer life than the lead storage cell. Thus, they are rechargeable.
(iii) Fuel cell uses Hydrogen and Oxygen to produce water molecules. The overall reaction is represented as:
2H2(g) + O2(g)→ 2 H2O(l)
It converts the energy of combustion into electrical energy. Oxygen is reduced at Cathode and the reaction involved is represented as:
O2(g) + 2H2O(l) → 4OH-(aq)
(iv) Mercury cell is a type of primary cell. The overall reaction of Mercury cell is represented as
Zn(Hg) + HgO(S) → ZnO(S) + Hg(l)
They do not involve any ion whose concentration varies during its lifetime and thus, the cell remains constant throughout its life. They are used for low current devices like in hearing aids, watches, etc.