What is the photoelectric effect? State the result of a photoelectric experiment that could not be explained on the basis of laws of classical physics. Explain this effect on the basis of quantum theory of electromagnetic radiations.
• When light radiation of an appropriate frequency (threshold frequency() falls on or hits a metal surface, the ejection of electrons from the metal surface takes place.
This ejection of electrons from the surface of a metal by radiations of suitable frequency is called the photoelectric effect (and the ejected electrons are called photoelectrons).
• The observations of the photoelectric effect experiment which could not be explained on the basis of classical physics (wave theory) theories are :
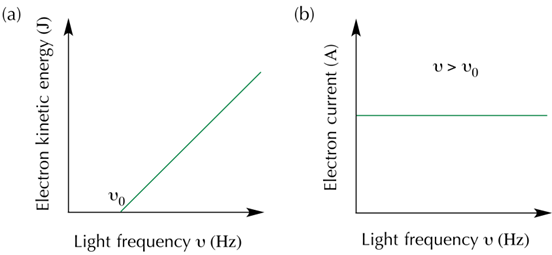
(i) First of all the kinetic energy possessed by the ejected electrons is directly proportional to the frequency of incident light and surprisingly is totally independent of the intensity of the incident light.
(ii) No electrons will be ejected from the metal surface unless and until the frequency of light used is greater than a specified minimum value i.e. threshold frequency (![]() .
.
• The classical wave theory fails to explain these phenomenon because classical physics tells that energy of light is proportional to the intensity of light, therefore by increasing intensity solely, it should have been possible to kick-start the emission of electrons and the energy (basically kinetic here) should have been proportional to the intensity of light
• But the actual observations contradict the classical point of view in this sense that the energy of the electrons does not even depend on the intensity, rather it depends on frequency.
• Photoelectric effect can be explained by the quantum concept of radiation given by Max Planck...
The observations of photoelectric effect experimentally provide with the following conclusions –
(i) The photocurrent is proportional to the intensity of incident radiation.
(ii) The magnitude of stopping potential and hence the maximum kinetic energy of emitted photoelectrons is proportional to the frequency of the emitted radiation.
(iii) There exists a minimum threshold frequency so that if radiation of frequency lesser than this threshold frequency is incident on the metal surface, there is no photoemission irrespective of the intensity of radiation.
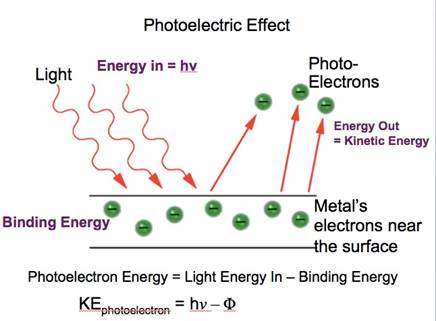
• Einstein explained (1905) the photoelectric effect on the basis of quantum theory, where the concept of quantized packets of energy i.e. photons (present in the light radiation and which are responsible for the emission of the electrons also )is introduced.
E=hν, where E is the energy of the incident photon (light radiation)
where
h is Plancks’s constant and ν is the frequency of radiation.
He proposed, that when a photon striking the metal surface it is using its binding energy (or (the energy which binds the electron to the nucleus) to eject the electron from the metal.
And the residual part of the energy is being transferred to the electron in the form of kinetic energy.
Therefore, the total energy of photon = binding energy + KE of the ejected electron
![]() (v= velocity of the ejected electron)
(v= velocity of the ejected electron)
or,![]() , where, v0 is the threshold frequency.
, where, v0 is the threshold frequency.
hence, kinetic energy of the ejected electron = h(v-v0)
this expression explains the experimental facts :
• If v> v0, the excess energy hv-hv0is transferred to the ejected electrons as kinetic energy.
Thus, with the increase in the frequency of the incident photon, the KE of ejected electron increases (Fig)
• If, v< v0, no electrons will be emitted whatever the intensity of the radiation is.
• There will be one electron ejected for each photon, which means increasing the intensity of light of o given frequency (> v0 ) number of photons striking the metal surface also increases., so as the numbers of electrons ejected. But the KE of electrons will be unchanged as long as a certain frequency.