Calculate the energy and frequency of the radiation emitted when an electron jumps from n = 3 to n = 2 in a hydrogen atom.
Electronic transitions in the hydrogen atom are given by the Rydberg’s equation:
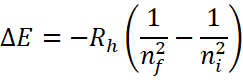
Where ![]() is the Rydberg constant.
is the Rydberg constant.
And ni and nf indicates the initial and final energy levels (n being the principal quantum number).
• For the given problem, ni implies to 3 and nf implies to 2
Hence,![]() =
= ![]()
=![]() × 0. 1389
× 0. 1389
= - 3.02802 × 10-19 J/H atom.
Here, energy is released or emitted.
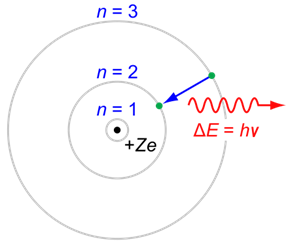
When it comes to the frequency of the photon emitted, the energy would be taken in terms magnitude only,
![]() , where, h= 6.626 × 10-34 J S, Planck’s constant and
, where, h= 6.626 × 10-34 J S, Planck’s constant and ![]() is the frequency of the radiation (energy) emitted (photon) due to the transition of the electron.
is the frequency of the radiation (energy) emitted (photon) due to the transition of the electron.
Hence, ![]()
= ![]()
=4.569 × 1014 S-1.