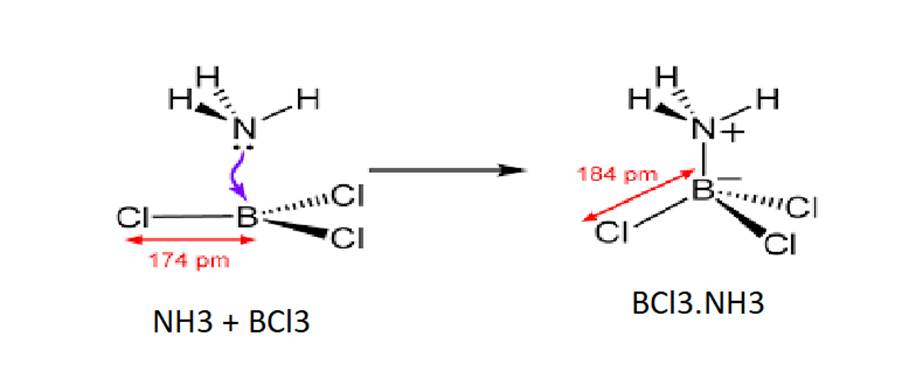
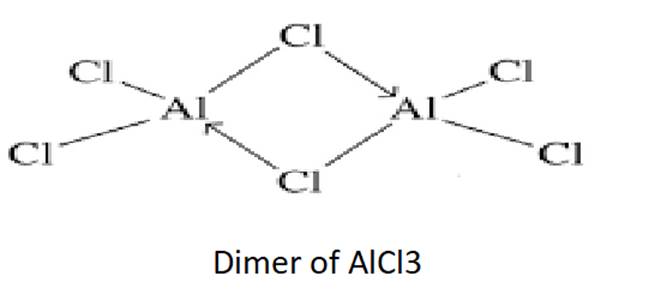
Draw the structures of BCl3.NH3 and AlCl3 (dimer).
The Lewis acid (BCl3) and the Lewis base (NH3) combine together to form a compound.
The structures of the BCl3.NH3is:
In AlCl3, Al has six electrons in the valence shell. Therefore, it is an electron-deficient molecule and needs two more electrons to complete its octet. The Cl atom has a lone pair of electron.
Thus, a molecule of AlCl3 combines with the other molecule of AlCl3 to form a dimer in order to complete its octet.
1