Draw the structure of boric acid showing hydrogen bonding. Which species is present in water? What is the hybridisation of boron in this species?
The central boron atom is connected to three hydroxyls (-OH) groups, which are capable of strong hydrogen bonding.
Its solid crystalline structure consists of parallel layers of boric acid held together in place by hydrogen bonds.
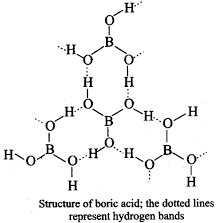
Hydrogen bonding in Boric acid
When boric acid reacts with water it forms ![]() species.
species.
![]()
The hybridization of Boron in this species is sp3.
1