Match the species given in Column I with the hybridisation given in Column II.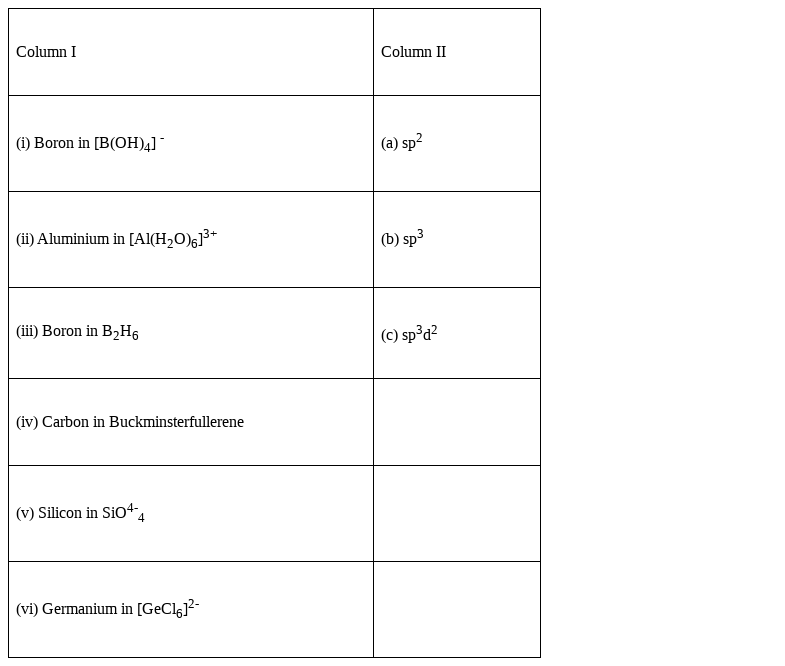
i) hybridisation of Boron in ![]() - b) sp3
- b) sp3
The EC of B is 1s2 2s2 2px1 2py0 2pz0. In the excited state, one of the electrons from 2s orbitals gets shifted to a p-orbital. The tetrahedral geometry is formed when hybridisation occurs between one s and 3 p-orbitals to give sp3 hybridisation.

Here, in this case 3 ![]() molecules bind to the 3 orbitals of the Boron, but still the octet of the Boron is not yet completely filled. Therefore, an extra molecule of
molecules bind to the 3 orbitals of the Boron, but still the octet of the Boron is not yet completely filled. Therefore, an extra molecule of ![]() binds to it. Thereby involving one s-orbital and 3 p-orbitals in the hybridization process. Thus, it is
binds to it. Thereby involving one s-orbital and 3 p-orbitals in the hybridization process. Thus, it is ![]() hybridised.
hybridised.
ii) Aluminium in [Al(H2O)6]3+ - c) sp3d2
The EC of Al is 1s2 2s2 2px2 2py2 2pz2 3s2 3px1. While in the excited state, one of the electrons from 2p and 3s orbitals gets shifted to 4s and 3d orbitals.

In other words, it can be said that in the excited state Al loses its 3 valence electrons (from the s and p-orbitals of the valence shell). Now, it accepts 6 electron pairs from water molecule and extended its orbital to d. Thus, forming sp3d2 hybridisation.
iii) Boron in ![]() - b) sp3
- b) sp3
The EC of B is 1s2 2s2 2px1 2py0 2pz0. In the excited state, one of the electrons from 2s orbitals gets shifted to a p-orbital. When hybridisation occurs between one s and 3 p-orbitals it gives sp3 hybridisation.
Normal state EC of B: 1s2 2s2 2px1 2py0 2pz0

Here, in this case 3 ![]() molecules bind to the 3 orbitals of one Boron. Similarly, 3 hydrogen ions are bonded with another Boron atom by making 1 s- and 3 p- orbitals. Thereby involving one s-orbital and 3 p-orbitals in the hybridization process in each case. Thus, it is
molecules bind to the 3 orbitals of one Boron. Similarly, 3 hydrogen ions are bonded with another Boron atom by making 1 s- and 3 p- orbitals. Thereby involving one s-orbital and 3 p-orbitals in the hybridization process in each case. Thus, it is ![]() hybridised.
hybridised.
iv) Carbon in Buckminsterfullerene – a) sp2
In this case, each carbon atom is sp2 hybridised while the remaining p- orbital available for bonding binds with another C-atom with sp2 hybridisation.
Normal state EC of C: 1s2 2s2 2px2 2py0 2pz0

In the case of Buckminsterfullerene, it is composed of 60 atoms of carbon (allotrope of Carbon), here, the hydrogen atoms hybridize with Carbon atoms while, the last p-orbital participates in hybridisation with the adjacent Carbon atoms. Thus, sp2 hybridisation.
v) Silicon in SiO4-4 - b) sp3
The EC of Si is 1s2 2s2 2p6 3s2 3p2. In the excited state, one of the electrons from 3s and 3p orbitals gets shifted to a p-orbitals. When hybridisation occurs between one s and 3 p-orbitals it gives sp3 hybridisation.
Normal state EC of Si: 1s2 2s2 2p6 3s2 3p2

In this case, due to the presence of vacant d-orbitals Si extends its coordination number and forms ![]() bonding and accommodates four atoms of oxygen.
bonding and accommodates four atoms of oxygen.
(vi) Germanium in [GeCl6]2-- c) sp3d2
The EC of Ge = [Ar] 3d10 4s2 4p2 4d0
EC of Ge (IV) [Ar] 3d10 4s0 4p0 4d0
Now, 6 Cl atoms donate 6 lone pairs of electrons to Ge which gets accommodated in 1 4s orbitals, 3 4p orbitalswhile the remaining 2 Cl atoms gets into the 4d orbitals thereby forming sp3d2 hybridisation.