Boron fluoride exists as BF3 but boron hydride doesn’t exist as BH3. Give reason. In which form does it exist? Explain its structure
pπ−pπ back bonding occurs in BF3.Boron has vacant 2p orbital and fluorine has completely filled 2p orbital so fluorine transfer 2p electron to boron. Now Boron’s deficiency has been filled making BF3 stable.
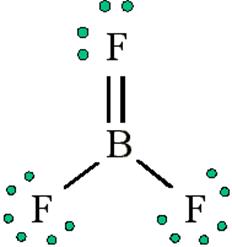
In BH3 hydrogen has lone pair and cannot fulfil the deficiency of boron so dimerise to form B2H6 which is in the shape of a banana and also known as a banana bond.
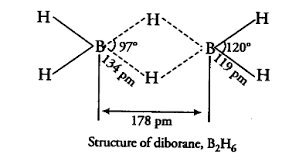
1