The value of Kc for the reaction 2HI (g) ⇋ H2 (g) + I2 (g) is 1 × 10-4
At a given time, the composition of the reaction mixture is
[HI] =2 × 10-5 mol, [H2] =1 × 10-5 mol and [I2] =1 × 10-5 mol. In which direction will the reaction proceed?
Given equation:![]()
Given, KC =1 × 10-4
By law of mass action the Equilibrium constant for the equation will be;
![]()
We know that the reaction quotient, Qc, expresses the relative ratio of products to reactants at a given instant.
![]()
[Qc = Reaction quotient]
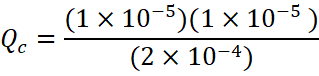
![]()
Here; QC >KC ![]() Reaction will proceed in reverse direction.
Reaction will proceed in reverse direction.
1