Match standard free energy of the reaction with the corresponding Equilibrium constant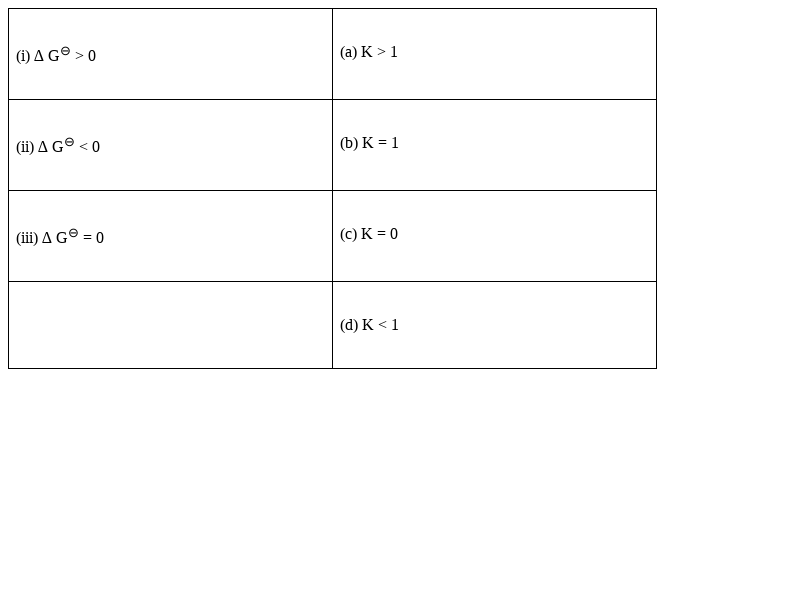
(i) ∆G0 > 0 - (d) K < 1
We know that,
∆G = ∆G° + RTln K… eq (1)
• ∆G = Gibbs free energy
• ∆G° = Standard Gibbs free energy (1atm pressure and 298k)
• T = temperature (in kelvin)
• R = Gas constant
• K = Equilibrium constant
• At Equilibrium, ∆G = 0
• ∆G° + RTln K = 0
So, RTln K = -∆G°...eq (2)
From the eq (2) we get smaller the magnitude of -∆G°, the higher the rate constant K will be and the faster the reaction.
Explanation: when the value of ∆G° is positive (+) the value of K will become negative hence, reaction will proceed in reverse direction i.e. non spontaneous reaction.
(ii) ∆G0 < 0 (a) K > 1
Explanation: when the value of ∆G° is negative (-) the value of K will become positive hence, reaction will proceed in forward direction i.e. spontaneous reaction.
(iii) ∆G0 = 0 (b) K = 1
Explanation: we know that, ∆G = ∆G° + RT ln K
At Equilibrium, ∆G = 0
From eq (1), we can write:
∆G° + RTln K = 0
So, RTln K = -∆G°
(∵ Log 1 = 0, so we get, RTln K = 0)
∴ k = 1
Hence value of K will become 1.