Suppose, the electron in a hydrogen atom makes transition from n = 3 to n = 2 in 10–8 s. The order of the torque acting on the electron in this period, using the relation between torque and angular momentum as discussed in the chapter on rotational mechanics is
Here, from Bohr’s quantization rule, we know angular momentum (L) is given by,
![]()
Where n=0,1,2….. and h=Planck’s Constant.
Now, the initial angular momentum is ![]()
And, the final angular momentum is ![]()
Now from the relation between angular momentum(L) and torque(τ), we have
![]()
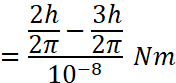
Putting ![]()
![]()
Hence, option (b) is the correct answer.
1