A beam of light having wavelengths distributed uniformly between 450 nm to 550 nm passes through a sample of hydrogen gas. Which wavelength will have the least intensity in the transmitted beam?
As we know that energy associated with a wavelength ![]()
Is equal to ![]() where
where ![]() is in nm and this is derived from
is in nm and this is derived from![]() .
.
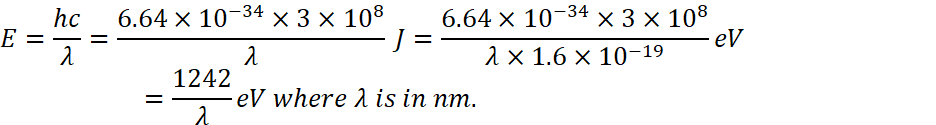
So,
Energy associated with 450nm wavelength ![]()
Energy associated with 550nm wavelength ![]()
Since the light is coming in the visible region.
So, we have n1=2 and n2=3,4,5,6….and so on.
Now energy corresponding to change in these transition state is
E2-E3![]()
E2-E4![]()
E2-E5![]()
As E2-E4 is in range between the two energy produced by two given wavelengths so wavelength corresponding to this energy is absorbs.
![]()
![]()
1