A beam of monochromatic light of wavelength λ ejects photoelectrons from a cesium surface (ϕ = 1.9 eV). These photoelectrons are made to collide with hydrogen atoms is ground state. Find the maximum value of λ for which
(a) hydrogen atoms may be ionized,
(b) hydrogen atoms may get excited from the ground state to the first excited state and
(c) the excited hydrogen atoms may emit visible light.
Since we have to find maximum ![]() so energy of ionization should be maximum.
so energy of ionization should be maximum.
So n1=1 and n2=![]()
![]() and we know that
and we know that
![]()
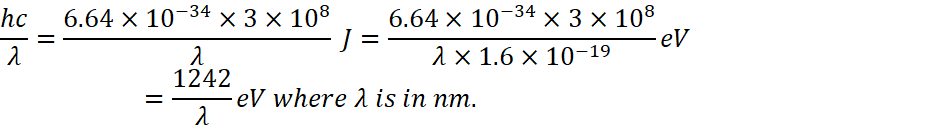
![]()
![]() eV
eV
![]()
(b)To change the transition state from n=1 to n=2
![]()
![]()
![]() eV
eV
![]()
(c) To get the light in visible region the change in transition state should be in Balmer series (n=2 to n=3)
But here atom is in ground state.
So, n=1 to n=3
Hence,
![]()
![]()
![]() eV
eV
![]()
1