Consider an excited hydrogen atom in state n moving with a velocity v (v << c). It emits a photon in the direction of its motion and changes its state to a lower state m. Apply momentum and energy conservation principles to calculate the frequency v of the emitted radiation. Compare this with the frequency v0 emitted if the atom were at rest.
As per the question ![]() is frequency of emitted radiation and
is frequency of emitted radiation and
![]() is frequency if atom is at rest and velocity of photon is u.
is frequency if atom is at rest and velocity of photon is u.
But as u << c so velocity of emitted photon is same as that of
Hydrogen atom =u.
Now applying Doppler’s effect formula
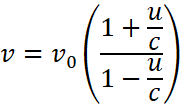
As u << c ![]()
Hence
![]()
1