Consider a photon of continuous X-ray coming from a Coolidge tube. Its energy comes form
The continuous energy spectrum of X-rays is a result of electrons striking the target atoms and loosing energy. An electron has a kinetic energy (K) given by K=eV, where “e” is the charge of the electron and “V” is the potential difference applied. Now, electrons can either give all of its energy to the target or only a fraction. So the generated photon can have any energy between “0” to “e” V. This gives us the continuous spectrum of wavelengths: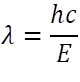
Here, λ is the wavelength of light, h is the Planck’s constant (6.62607004 × 10-34 m2 kg / s), c is the speed of light in vacuum and E is the energy of the photon.
1