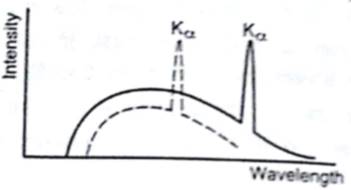
Figure shows the intensity-wavelength relations of X-rays coming from two different Coolidge tubes. The solid curve represents the relation for the tube A in which the potential difference between the target and the filament is VA and the atomic number of the target material is ZA. These quantities are VB and ZB for the other tube. Then,
The cut-off frequency is given by:
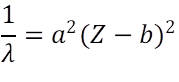
![]()
where, λmin is the cut-off wavelength, h is the Planck’s constant (6.62607004 × 10-34 m2 kg / s), c is the speed of light in vacuum, e is the charge of the electron and V is the potential difference applied at the ends of the tube
So, greater the operating voltage, lesser would be the cut-off wavelength. As, λmin is less of A than B, VA > VB .
From Moseley’s law, √v = a (Z – b), therefore in terms of wavelength(λ ), we have
where, “a” represents the proportionality constant while “b” represents the screening constant and Z is the atomic number of the element.
Looking at the characteristic peaks, we can see that λ A >λB . So from the inverse relation, ZA<ZB .