Mark the correct options.
An atom with vacancy has higher energy than a neutral atom. So, (A) is incorrect.
K X-ray is created when a vacancy (hole) is filled in the K shell by an electron jumping from the successive shell to k-shell. So, the hole or vacancy is created in the shell where the electron jumps from. Hence, option (B) is correct.
Characteristic K X-rays are produced when an electron from subsequent L, M, and N… shells drops to fill the vacant K shell. The energy of this transition is greater than the energy of transition form M, N, O… to L shell. Wavelength is inversely promotional to energy. So, KX rays have shorter wavelengths.
The wavelengths for K and K are given as:
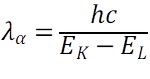
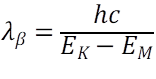
where, λα and λβ are the wavelength, h is the Planck’s constant(6.62607004 × 10-34 m2 kg / s), c is the speed of light in vacuum, EK-EM and EK-EL are the difference in energy levels between the K and M shells and K and L shells.
As, EK-EM is more than EK-EL, λα >λβ. So, (D) is incorrect.