A certain element emits Kα X-ray of energy 3.69 keV. Use the data from the previous problem to identify the element.
Given:
Energy of Kα X-rays= 3.69 KeV or 3690 eV
we’re asked to identify the element which exhibits this behavior,
Wavelength is given by
![]()
![]()
Using Moseley’s law √c/![]() = α(Z – b),
= α(Z – b),
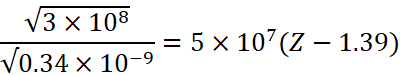
![]()
![]()
![]()
Which gives us,
![]()
Z=20.17 (atomic number) which can be approximated to Z=20
Therefore, the element with z=20 is calcium (Ca)
1