Atmospheric pressures recorded in different cities are as follows: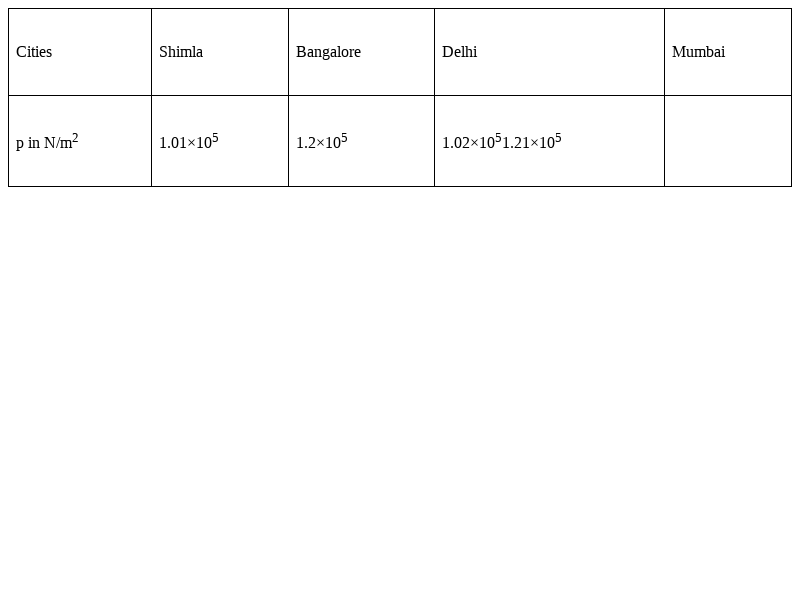
Consider the above data and mark the place at which liquid will boil first.
There are 2 conditions for liquefaction of gases:
• Low temperature
• High pressure
LOW TEMPERATURE: As the temperature of a gas is low, the kinetic energy of the molecule also decreases, which leads volume to get decrease. As the temperature decreases, molecules come close to each other and changes into a liquid.
HIGH PRESSURE: As the pressure increases, makes gas molecule come close to each other and convert into liquid. For each gas, there is a specific temperature above which gas never be liquefied, however, high pressure is applied.
Therefore the effect of temperature is more important than that of pressure.
Shimla has the lowest atmosphericpressure so liquid will boilfirst there.The atmospheric pressure is very low at high altitudes, so at very low temperature, the vapour pressure of the liquid becomes equal to atmospheric pressure i.e. below boiling point. Liquid starts to boil much before the food is cooked.