Match the graphs between the following variables with their names: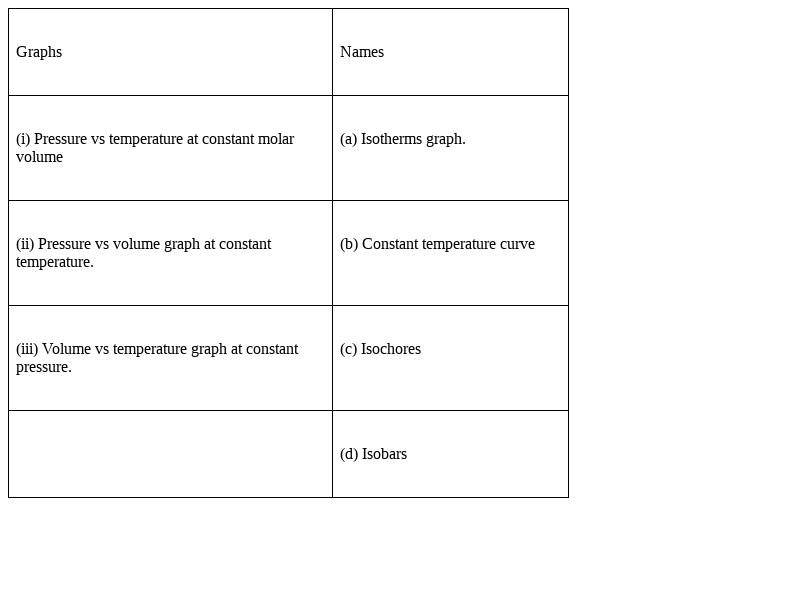
(i)-(c)
(ii)- (a), (b)
(iii)- (d)
(i) Pressure vs temperature at constant molar volume is known as Isochores, as Isochore means a line on a graph plotted for the variation of the temperature of any gas with its pressure,keeping volume constant.
Hence, the term used for Pressure vs temperature at constant molar volume graph is Iscochores.
(ii)Pressure vs volume graph at constant temperature is known as an Isotherms graph or Constant temperature curve, this is because we know that the word “Isothermal” means temperature remains constant for that particular system and if we are taking about any process in which no change in temperature occurs during the course of the reaction the process is called "isothermal process".
Hence, when we plot Pressure vs volume graph at constant temperature it is called an Isotherms graph.
(iii) Volume vs temperature graph at constant pressure is known as Isobars.
As we all know that any system having constant pressure, is known as the isobaric system, and when we plot Volume vs temperature graph keeping pressure constant, it is better to be known as Isobaric graph.