Match the following gas laws with the equation representing them.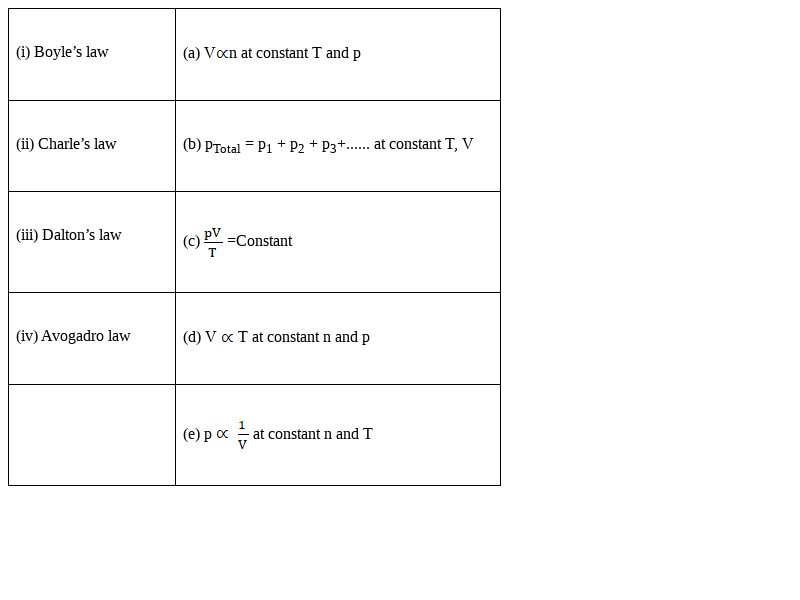
(i) – (e)
(ii) – (d)
(iii) – (b)
(iv) – (a)
(i)Boyle’s law states that “at constant temperature, the pressure of a fixed amount of gas(n moles)varies inversely with its volume.Which means that if we increase the pressure, the volume of the gas will be decreased and it is understandable by the example of LPG Gas cylinder in which so high pressure is maintained so that the gas liquefied and attain very small volume of cylinder.
If we write Boyle’s law mathematically, we get:
p![]() at constant n and T
at constant n and T
Where p = pressure of a gas
V = Volume of the gas
n = fixed amount of gas
T = constant temperature
(ii) Charle’s law states that “pressure remaining constant, the volume of a fixed mass of a gas is directly proportional to its absolute temperature”.
If we increase the temperature the Volume of gas increases.
If we write Charle’s law mathematically, we get:
V![]() T at constant n and p
T at constant n and p
Where p = pressure of a gas
V = Volume of the gas
n = fixed amount of gas
T = constant temperature
(iii) Dalton’s law states that “the total pressure exerted by the mixture of non-reactive gases is equal to the sum of the partial pressure of individual gases”.
We know that the pressure exerted by individual gas in the mixture is called partial pressure of that gas.
So, if we write the Dalton’s law mathematically, we get:
p Total = p1 + p2 + p3+...... at constant T, V
Where p1, p2, p3….etc are the partial pressure of gas 1, gas 2, gas 3 ……respectively.
p Total = the total pressure exerted by the mixture of non-reactive gases.
V = Volume of the gas
T = constant temperature
(iv) ) Avogadro law states that “the equal volumes of all gases under the same conditions of temperature and pressure contains equal number of molecules”.
It means 1 L of any two gas at same T and p, will contain same number of moles of that two different gases, irrespective of their molar mass etc.
It can be written mathematically as:
V ![]() n at constant T and p
n at constant T and p
Where V = Volume of the gas
T = constant temperature
n = fixed amount of gas
p = pressure (constant)