The entropy change can be calculated by using the expression
ΔS = . When water freezes in a glass beaker, choose the correct statement amongst the following:
. When water freezes in a glass beaker, choose the correct statement amongst the following:
Freezing is an exothermic release so there will be release in energy, which is absorbed by surrounding.
ΔS (system)=-  and ΔS (surroundings)=
and ΔS (surroundings)=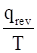
Therefore, the entropy of system will be decrease and that of surrounding will be decrease.
1