On the basis of thermochemical equations (a), (b) and (c), find out which of the algebric relationships given in options (I) to (iv) is correct.
(a) C (graphite) + O2 (g) → CO2 (g); ΔrH = x kJ mol–1
(b) C (graphite) +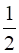 O2 (g) → CO (g);ΔrH = y kJ mol–1
O2 (g) → CO (g);ΔrH = y kJ mol–1
c) CO (g) + 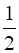 O2 (g) → CO2 (g);ΔrH = z kJ mol–1
O2 (g) → CO2 (g);ΔrH = z kJ mol–1
Reason:
C (graphite) + O2 (g) → CO2 (g); ΔrH = x kJ mol–1..…(i)
C (graphite) +  O2 (g) → CO (g);ΔrH = y kJ mol –1……(ii)
O2 (g) → CO (g);ΔrH = y kJ mol –1……(ii)
CO (g) +  O2 (g) → CO2 (g);ΔrH = z kJ mol–1 ……(iii)
O2 (g) → CO2 (g);ΔrH = z kJ mol–1 ……(iii)
Adding (ii) and (iii) will be result in equation (I)
Which means that y + z = x
1