For an ideal gas, the work of reversible expansion under isothermal condition can be calculated by using the expression w = – nRT ln 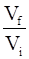
A sample containing 1.0 mol of an ideal gas is expanded isothermally and reversibly to ten times of its original volume, in two separate experiments. The expansion is carried out at 300 K and 600 K respectively. Choose the correct option.
Reason:
As we all know the below relation Vf
w = -nRT ln
Here R, T, and ln are the same in both cases, sowork done will only depend on the temperature.
are the same in both cases, sowork done will only depend on the temperature.
 =
=  =
=  = 2
= 2
Here w2 indicates work done at 600K
w1indicates work done at 300k
so, from the above expression, it is clear that work done at 600K is twice the work done at 300k.
(iv)Internal energy depends on the temperature and it is given that the above process is isothermal, which indicates ΔU=0, in both the cases.