What will be the work done on an ideal gas enclosed in a cylinder, when it is compressed by constant external pressure, pext in a single step as shown in Fig? 6.2. Explain graphically.
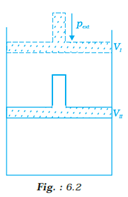
o Work done in thermodynamics is obtained at constant pressure by the relation W=p∆v, i.e. the product of the constant pressure and the change in volume.
o Now, if we plot P(y-axis) vs V (x-axis ) graph for the given process, then we get the following figure :
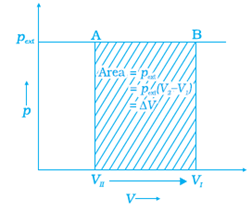
From this graph we can obtain the be the work done on the ideal gas enclosed in the cylinder in 1 step: the area covered by P-V graph (shaded region) is the actual value of the work done is:
= length × breadth = pext ∆V = AVI (or BVII ) × (VI - VII )
1