Enthalpy diagram for a particular reaction is given in Fig. 6.3. Is it possible to decide the spontaneity of a reaction from the given diagram. Explain.
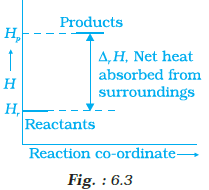
It is not possible to take a decision on whether the given reaction will be spontaneous or not.
o From the given enthalpy diagram it can be said the change in enthalpy ∆H is positive for the reaction, i.e. it will be endothermic in nature.
o But when comes to the spontaneity of a reaction, enthalpy is just a factor and there are other important factors like entropy, Gibb's free energy also taken into consideration.
Hence, enthalpy alone cannot determine the spontaneity of a reaction; one must have a loo to the other contributing factors also.
1