Match the following :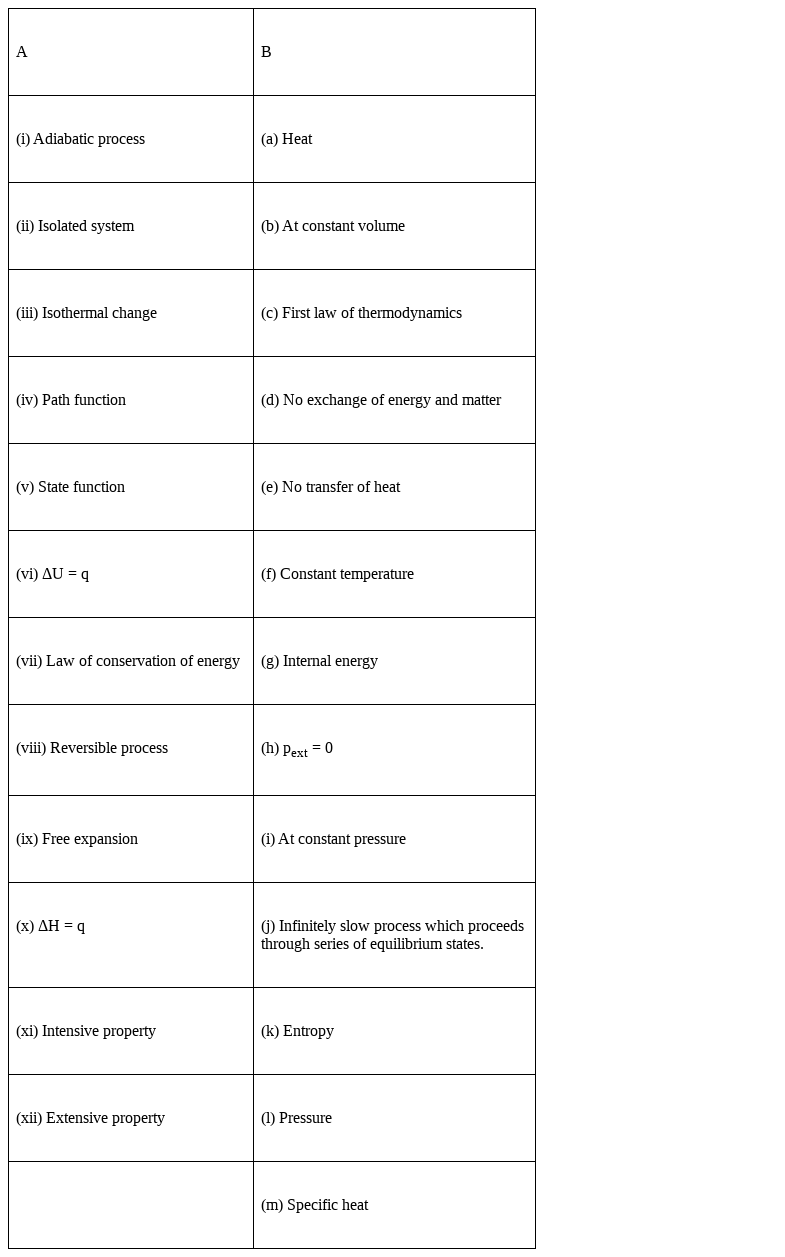
(i) Adiabatic process – (e) No transfer of heat
Explanation: The process in which there is no transfer of heat between system and surrounding is known as an adiabatic process. The barrier or wall separating the system is called an adiabatic wall.
(ii) Isolated system – (d) No exchange of energy and matter
Explanation: The system in which there is no exchange of energy and matter between system and surrounding is known as an isolated system. Example: Tea in a thermos flask.
(iii) Isothermal change – (f) constant temperature
Explanation: When there is a slow transfer of heat from the system to surrounding that the thermal equilibrium will get maintained is an isothermal change.
(iv) Path function – (a) Heat
Explanation: Path function transit between initial to final state and work and heat are two common path functions.
(v) State function - (g) Internal energy, (k) Entropy , (l) Pressure
Explanation: state function depends only on the initial and final state and not on the path of the system. It depends on measurable properties like pressure, volume, temperature, enthalpy, entropy, mass, density.
(vi) ΔU = q - (b) at constant volume
Explanation: FREE EXPANSION
When the expansion of the gas takes place in a vacuum, it is called free expansion. No work is done of an ideal gas during free expansion whether the process is reversible or irreversible.
we know that ∆U = q + w ---(1)
Also, W= -p∆V
Substitute the value of W in equation (1)
∆U = q - p∆V
If the process is carried out at constant volume(∆V = 0)
∆U = qv
The v in qv denotes the heat is supplied at constant volume.
(vii) Law of conservation of energy- First law of thermodynamics
Explanation: First law of thermodynamics state that energy of an isolated system is constant. It also based on conservation law where energy can neither be created nor be destroyed.
(viii) Reversible process – (j) infinitely slow process which proceeds through series of equilibrium.
Explanation: Reversible process is the process which takes infinite time by doing a series of steps so that system and surrounding remain in equilibrium and if it brought back to initial state there is no effect on surrounding.
(ix) Free Expansion – (h) pext = 0
In Vaccum expansion of gas ( free expansion) pext = 0 . In an ideal gas, whether the process is reversible or irreversible, work done during free expansion is zero
wirreversible= -p∆V
= 0×∆V
wirreversible = 0
∆H = q – (i) At constant pressure
Explanation: From the first law of thermodynamics
∆U = q + W --- (1)
∆U= internal energy
q= heat
W= work done
We know W = -p∆V ---(2)
Therefore we can write eq-1 as
∆U = q - p∆V --- (3)
→ q is the heat absorb at constant pressure
Now if the system absorb q (heat), internal energy changes from U1 to U2 and volume increase from V1 to V2
∆U = U2– U1 –-- (4)
∆V = V2 - V1 --- (5)
Put equation (4) and (5) in equation (3)
U2 - U1= q - p (V2 - V1)
qp = U2 - U1+ p (V2 - V1) --- (6)
U, P, V are state functions and U + PV is heat content or enthalpy of the system which is denoted by
H = U + PV –-- (7)
So, q = H2 – H1
q = ∆H
This is the heat absorbed at constant pressure.
(xi) Intensive property – (a) heat, (l) pressure, (m) specific heat
Explanation: those properties which depend on the nature of the substance and not the quantity of the system are known as an intensive property.
(xii) Extensive property – (g) internal energy, (k) entropy
Explanation: Those properties which depend on the quantity of the system are known as extensive property.