Match the following processes with entropy change: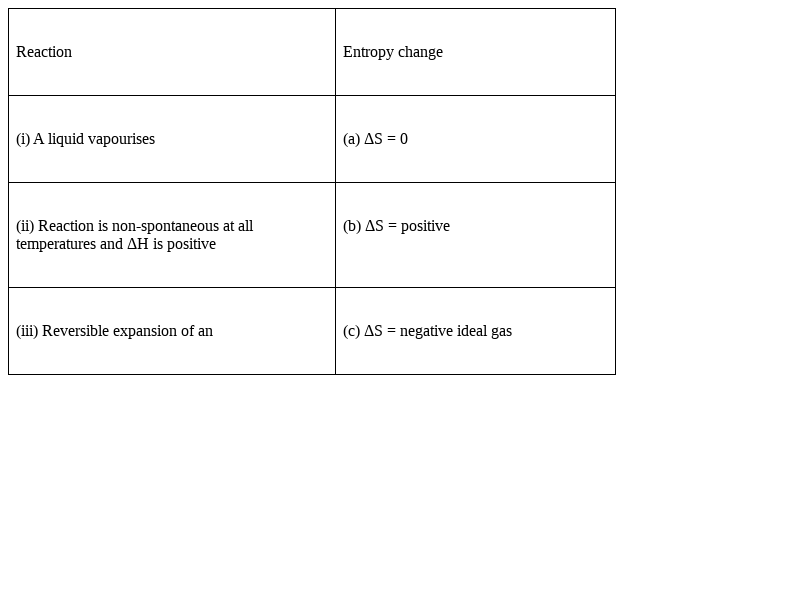
(i) A liquid vaporize – (b) ΔS = positive
Explanation: When the liquid vaporizes, the degree of the disorder increases, therefore, entropy is always positive.
(ii)The reaction is nonspontaneous at all temperatures and ΔH is positive – (c) ΔS = negative ideal gas
Explanation: When the reaction is non-spontaneous
∆Suniverse˂ 0
If a reaction has positive entropy and enthalpy change, the process can be spontaneous when T∆S is more enough to overweigh ∆H.
a. Entropy change in the system can be small in which Temperature must be large.
b. Entropy change in the system can be large in which temperature must be small
Because of the above reasons, reactions are often carried out at high temperature.
(iii) Reversible expansion of an - (a) ΔS = 0
Explanation: When the system is at equilibrium or reversible, the entropy remains constant
ie why ΔS = 0