Match the items in Column I with relevant items in Column II.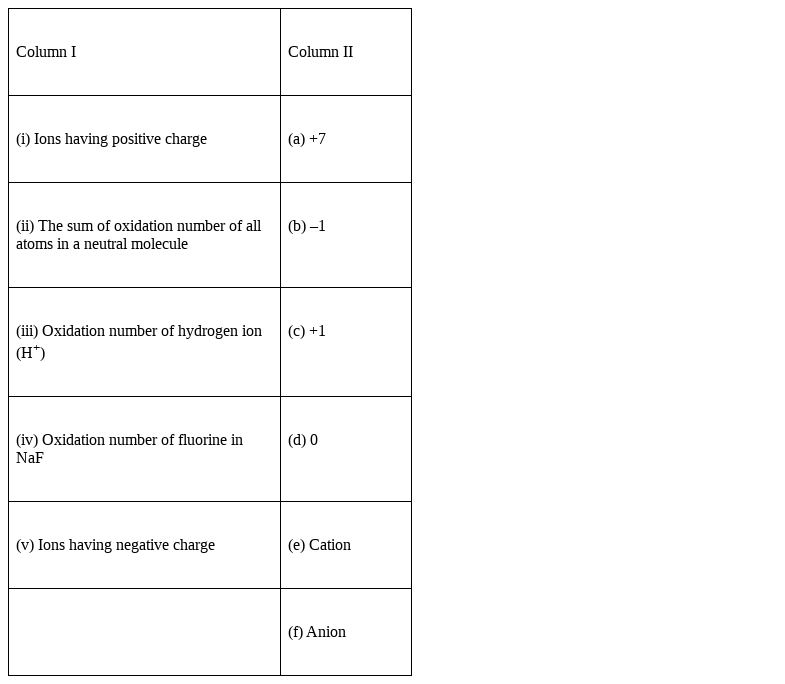
Option (i) Ions having positive charge → (e) Cation
Cations are positive charge entities in ionic compounds.
Option (ii) the sum of oxidation numberof all atoms in a neutral molecule → (d) 0
The algebraic sum of all oxidation numbers of a neutral compound is equal to 0. For ionic compounds
Option (iii) Oxidation number of hydrogen ion → (c) +1
Hydrogen atom is +1 charge for bonding with no9n-metals, whereas its oxidation number is -1 with metals is -1, for example metal hydrides.
Option (iv) Oxidation number of F in NaF → (b) -1
For all compounds of fluorine the oxidation state is -1 as it is most electronegative element in the entire periodic table.
Option (v) Ions having negative charge → (f) anion
By definition the ions having negative charge are called anion.