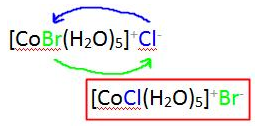Due to the presence of ambidentate ligands coordination compounds show isomerism. Palladium complexes of the type [Pd(C6H5)2(SCN)2] and [Pd(C6H5)2(NCS)2] are
Ligands that have two different bonding sites are called ambident ligands. Few examples are SCN-,NO2 etc…
SCN-→ Metal or NCS→Metal
So, [Pd(C6H5)2(SCN)2] and [Pd(C6H5)2(NCS)2] are linkage isomers because they differ in the binding site of the ligands which leads to different linkage.
Whereas, a coordination isomer is a form of structural isomerism in which the composition of the complexion varies. In a coordination isomer the total ratio of ligand to metal remains the same, but the ligands attached to a specific metal ion change.
For Example: A solution containing ([Co(NH3)6]3+) and [Cr(CN)6]3- is a coordination isomer with a solution containing ([Cr(NH3)6] and [Co(CN)6]).
Ionisation Isomers: These are identical except for a ligand has exchanged places with an anion or neutral molecule that was originally outside the coordination complex. The central ion and the other ligands are identical.