Atomic number of Mn, Fe and Co are 25, 26 and 27 respectively. Which of the following inner orbital octahedral complex ions are diamagnetic?
Strong field ligands cause pairing of electrons
Electronic configuration of
1) Co3+ - [Ar]
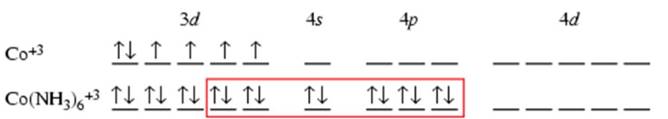
Number of unpaired electrons =0
So, [Co(NH3)6]3+ is diamagnetic.
2) Mn3+
As, Mn forms low spin compound with CN lignad, so
[Ar] 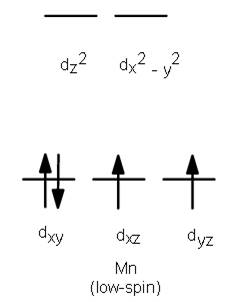
Number of unpaired electrons=2
So, [Mn(CN)6]3- is paramagnetic
3) Fe2+
[Ar] 3d4 4s0 and in d orbital there will be pairing because CN is a strong ligand.
Number of unpaired electrons=0
So, [Fe(CN)6]4- is diamagnetic
4) Fe3+
[Ar] 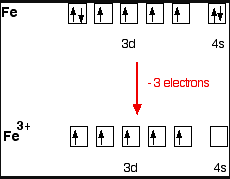
Number of unpaired electrons =1 because as 3d orbital is half filled and we all know that half filled orbital is more stable than pairing.
So, [Fe(CN)6]3- is paramagnetic
1