Match the complex species given in Column I with the possible isomerismgiven in Column II and assign the correct code :
Column I (Complex species) Column II (Isomerism)

Code:
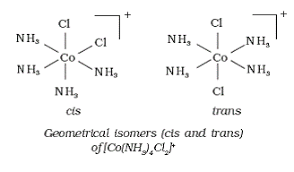
• A.[Co(NH3)4Cl2]+ shows geometrical isomerism due to different geometric arrangements of the ligands.
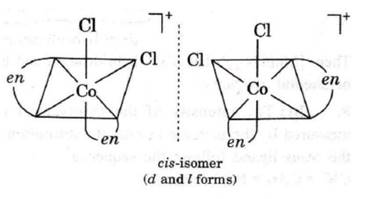
• B Cis-[Co(en)2Cl2]+ shows optical isomerism because the two (Dextro d and laevo l) mirror images formed cannot be superimposed on one another.
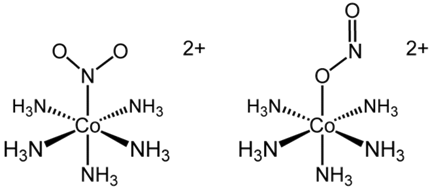
• C.[Co(NH3)5(NO2)]Cl2 shows linkage isomerism because it contains ambidentate ligand NO2.
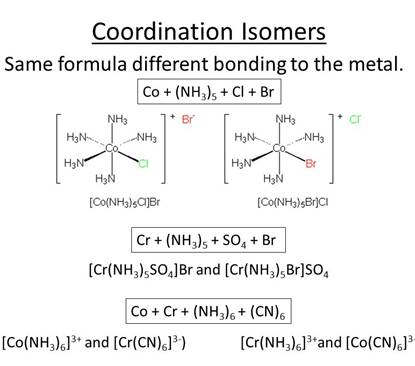
• D.[Co(NH3)6][Cr(CN)6] shows coordination isomerism due to interchanging of ligands between ionic entities.
The correct answer will be the option (iii) A (4) B (1) C (5) D (3).
Hence, matching the two columns the right combination will be:
A. [Co(NH3)4Cl2]+ -4. geometrical
B. Cis-[Co(en)2Cl2]+-1. optical
C. [Co(NH3)5(NO2)]Cl2-5. linkage
D. [Co(NH3)6][Cr(CN)6] -3. Coordination.