Match the compounds given in Column I with the oxidation state of cobalt present in it (given in Column II) and assign the correct code.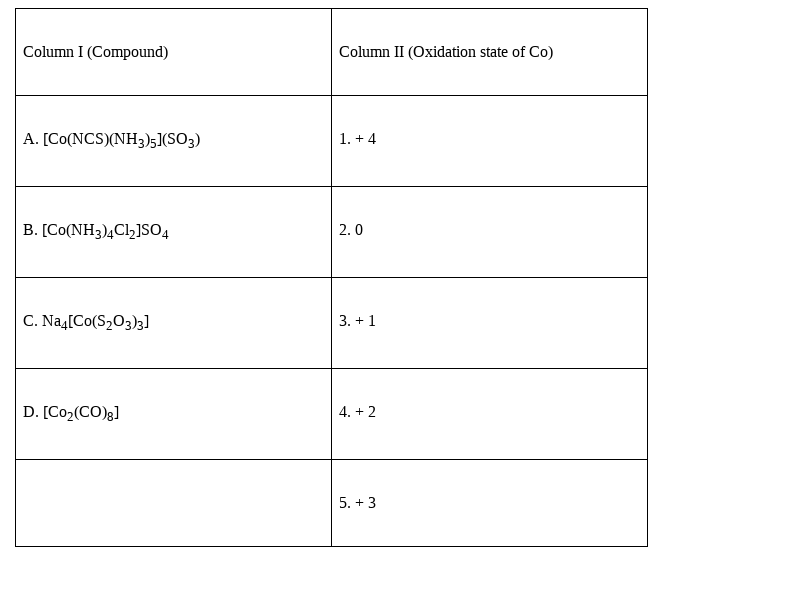
Code:
The correct answer will be the option (iii) A (5) B (1) C (4) D (2)
Hence, matching the two columns the right combination will be:
A. [Co(NCS)(NH3)5](SO3) -5. + 3 (oxidation no.of Co, x =-1![]() (-1)-(-2)=+3)
(-1)-(-2)=+3)
B. [Co(NH3)4Cl2]SO4 -1. + 4 (oxidation no. Of Co, x =-2x(-2)-1![]() (-2)=+4)
(-2)=+4)
C. Na4[Co(S2O3)3] -4.+ 2 (oxidation no. Of Co, x=-3![]() (-2) – 4x(+1)=+2 )
(-2) – 4x(+1)=+2 )
D. [Co2(CO)8] –2. 0 . (as CO is a neutral ligand, therefore Co here has a zero oxidation state)
1