Note: In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
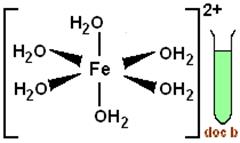
Assertion: [Cr(H2O)6]Cl2 and [Fe(H2O)6]Cl2 are reducing in nature.
Reason: Unpaired electrons are present in their d-orbitals.
Assertion and reason both are true, but the reason is not the correct explanation of assertion.
In [Cr(H2O)6]Cl2 the oxidation state of central metal atom Cr is x=-(-2)=+2 and for [Fe(H2O)6]Cl2 also the oxidation state of Fe is +2. Hence, both of them are in their lower oxidation states and that means they can further be oxidised and therefore they are reducing in nature. Also, they have enough d-electrons to donate to other ligands and have more compounds formed.
And the corresponding structures are:
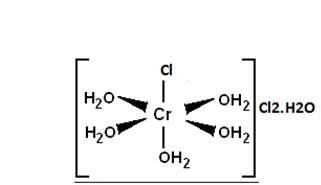 and
and