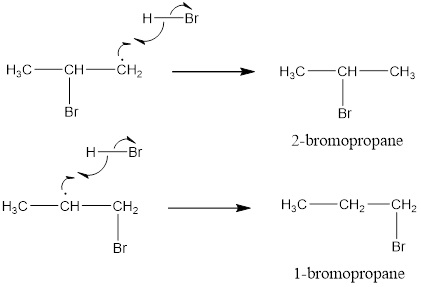
Predict the major product (s) of the following reactions and explain their formation.

Reaction (i) takes place as follows anti-markonikows rule due to presence of peroxide and gives a primary aklyk halide as compared to a 2° alkyl halide, which proceeds by free radical mechanism.
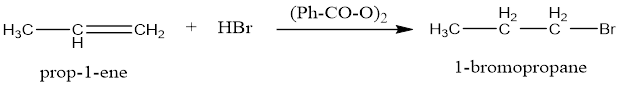
Step 1: Homolysis of peroxide to from free radicals
 The carbonyl free radical is less stable as compared to phenyl radical, so it further reduces to give out CO2 in the process. This free radical initiates the breaking of double bond in alkenes.
The carbonyl free radical is less stable as compared to phenyl radical, so it further reduces to give out CO2 in the process. This free radical initiates the breaking of double bond in alkenes.
Step 2: Formation of Bromine free radical
![]()
This bromine radical induces homolytic breaking of double bond to give mixture of primary and secondary free radical.
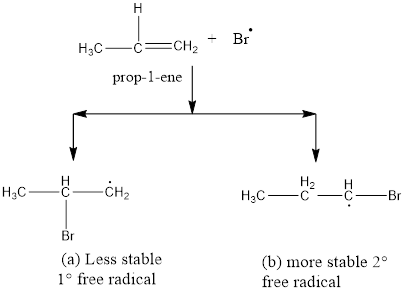
Step 3: reaction of hydrogen bromide with alkyl radical
Final step includes the proton addition to the alkyl radical. This step is also called as chain terminating step, also the bromine radical required for further process is produced at the end of chain terminating step. Due to the chain growth mechanism these reactions are difficult to limit and produce multiple derivatives if the stearic hindrance of bromine is below the threshold limit.